Explain How Acid-base Strength Is Different From Concentration
BProvide or use representations of. A dilute acid solution is not the same as a weak acid solution and a concentrated acid solution is not the same as a strong acid solution.
That means it depends on the concentration of H plus ions.

. Similarly an acid-base reaction under the Bronsted-Lowry definition entails the transfer of a proton from an acid to a base. Compaced PH Part 3. Concentration refers to the number of moles per volume are contained within the solution.
Converesly the concentration be very low acid. Up to 24 cash back The concentration of an acid or base is the amount of moles per unit volume. Unlike the K a value the percent ionization of a weak acid varies with the initial concentration of acid typically decreasing as concentration increases.
While the concentration of the strong acid at equilibrium is zero and the concentration of the conjugate. On molecular level some of the acid will still be present as the acid partially ionizes The concentration of the weak acid at equilibrium is higher than the concentration of the conjugate base and H3O. Acid concentration and strength - Higher A solution forms when a solute dissolves in a solvent.
The Bronsted-Lowry definition of acids and bases defines an acid as a proton donor and a base as a proton acceptor. In a competition reaction between two bases for the same acid one must consider both the relative strength of the bases and. The difference between the strong acid and the weak acids are given below.
Question A solution of hydrochloric acid with a concentration of 2 gdm 3 has a pH of 13. Factors Affecting AcidBase Strength. We will see words like CONCENTRATED and DILUTE.
A strong acid does not become a weak acid just because it is diluted. Strong baseA strong base is a basic chemical compound that is able to deprotonate very weak acids in an acid-base reaction. Strength- The amount of HOH- ions the acidbase is able to givereceive strength is measured through the ph scale which is from 0-14 Concentration- The amount of.
A strong acid always has a higher acid strength. It also applies to how. Up to 24 cash back I am talking about the strength and concentration of acids and bases.
The student directions work for both the Java and html5 sims. Strong acids ionize completely in an aqueous solution. Rate of reaction is faster.
It always loses H when dissolved in water. A base is an aqueous substance that could absorb hydrogen ions. The higher the concentration of H plus ions the more acidic the solution and the lower the concentration of H plus ions the less acidic the solution.
Weak acid as compared to the same concentration as strong acid will have. An acid or a base may be hard or soft and also be either weak or strong. A concentrated acid may or may not have a high acid strength.
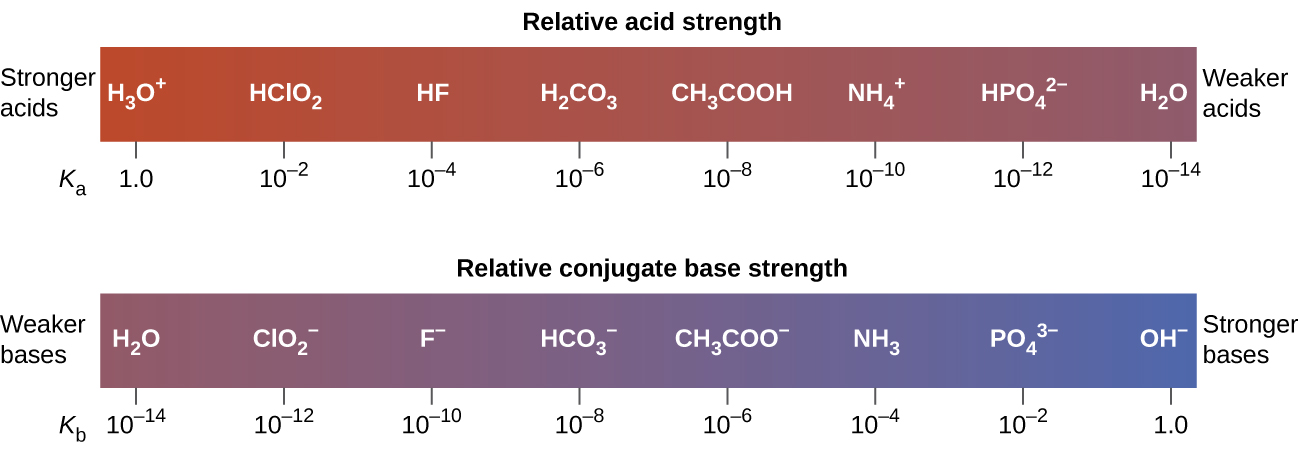
Weaker bases have smaller K b values. Remember that acid strength depends upon Ka not upon analytical concentration. Watch for updated clicker question images.
This picture shows two beakers with the same solution in it but one is in a smaller beaker. Explain how the pH at the equivalence point varies as the strength of the acid increases. A base dissociation constant K b mathematically represents the bases relative strength and is analogous to the acid dissociation constant.
In solutions of the same concentration stronger bases ionize to a greater extent and so yield. You can tell the difference between a strong acid and a weak acid. Relies on the concentration of the hydronium ions.
Concentrated ethanoic acid and dilute ethanoic acid are both weak acids because they are only partly ionised in water. Acid Base Solutions - Concentration and Strength. Common examples of strong bases are the hydroxides of alkali metals and alkaline earth metals such as NaOH and CaOH 2Very strong bases are even able to deprotonate very weakly acidic CH groups in the absence of water.
AIn both words and in terms of a mathematical equation describe the relationship between pH and the ratio of conjugate base to acid concentration that you observed in this experiment. It refers to how much of an acid the solute is dissolved in the solution. Like weak acids weak bases can be used to make buffer solutions.
The concentration of a solution is a measure of how crowded the solute particles are. The strength of an acid or base refers to how much of the acid or bases ions are released in a solution. Concentrated hydrochloric acid and dilute hydrochloric acid are both strong acids because they are both completely ionised in water.
This is completely different that strength of the solution. A proton acceptor that does not ionize fully in an aqueous solution. In organic chemistry acid-base reactions are ubiquitous.
Adding more of the solvent eg water reduces the concentration making the solution more dilute. Concentration is the amount of solute something that dissolves that is dissolved in a certain amount of solvent usually water Dissolving more of the substance into a given volume increases the concentration. A strong acid or base completely ionizes in a solution while weak acid or base only partially ionizes in a solution.
And similarly the basic strength of a solution it depends on the concentration of OH minus ions. Your answer should be based upon the proton transfer reaction that is important at the. AcidBase Strength It is important to realize that hardsoft considerations have nothing to do with acid or base strength.
This means that if a solution has higher molarity than its concentration is higher as well. Note that the strength of an acid is not affected by its concentration. Concentration of Acids is different.
Up to 256 cash back Include a brief fewer than 4sentence explanation foreach ofthe followingthat cites raw data or information gathered from plots. Relies on the concentration of the hydroxide ions. It possess high conductivity due to the presence of the unpaired atoms.
A concentration acid contains the maximum amount of solutes per unit volume of solution at a given temperature. Acid Base titration question. This is because depends on the concetration of the hudcoyen ions His a good way 8t a of the Strong acid Should Weak.
A strong acid does not contain the maximum amount of solutes per unit volume. Students will be able to aGenerate or interpret molecular representations words andor pictures for acid or base solutions. The hydrogen ion concentration decreases by a factor of 10 so the pH increases by 1 from 16 to 26.
Explain the difference between equivalence point and end point. An acid is any chemical compound once dissolved in water produces a solution with hydrogen ion activity more than purified water. It contains ionic bonds.
Acid Strength pH and Ka 7. Just as for acids the relative strength of a base is reflected in the magnitude of its base-ionization constant K b in aqueous solutions. Up to 24 cash back Strength.
There is a difference between the strength of an acidbase and the concentration of an acidbase.

Strong Acids And Strong Bases Acids And Bases That Are Strong Electrolytes Completely Ionized Chemistry Education Teaching Chemistry Organic Chemistry Study

Comments
Post a Comment