Describe the Properties of Ionic Compounds
Advertisement Answer 0 BrainlyHH. Ionic Compounds have high boiling and melting points as theyre very strong and require a lot of energy to break.

6 1 Ionic Compounds Pages Learning Goals I Can Explain How Ions And Ionic Compounds Are Formed I Can Describe The Properties Of Ionic Compounds Ppt Download
One of the ions has a positive charge called a cation and the other has a negative charge anion.

. There are many properties. Properties of Ionic Compounds Ionic Compounds have high boiling and melting points as theyre very strong and require a lot of energy to break. In solution they.
Ionic compounds are solids are somewhat hard because of strong force of attraction between the positive and negative ions. They have high melting point and also high boiling points. They have higher enthalpies of fusion and vaporization than molecular compounds.
The hydration of ions by water molecules liberates heat energy. The electrostatic forces of attraction between oppositely charged ions lead to the formation of ions. Using at least 2 properties of ionic compounds explain why is cookware not made from ionic compounds.
The charges on the ions in ionic compounds cause the cations and ions to be tightly bound to one another. Ionic compounds are solids These compounds have high boiling and melting points. High melting points High boiling points They are generally hard and brittle They are electrically neutral They form efficient insulators High enthalpies of fusion thermal energy needed to melt one mole of the solid form and vaporization thermal energy needed to vaporize one mole of the liquid form.
Form when metals react with nonmetals exist as crystalline solids hard and brittle high melting points because the attractive forces between ions intramolecular forces are stronger high boiling points because the attractive forces between ions are stronger. When electricity flows through an ionic compound the ions. Ionic bonds are strong.
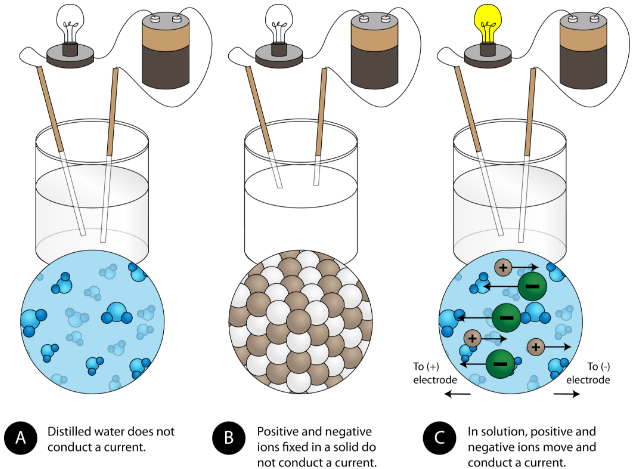
They conduct electricity but only when they are dissolved in water. Ionic compounds are generally soluble in water insoluble in solvents such as kerosene petrol etc 4. Describe the properties of ionic compounds Question The strong attraction between ions that make up an ionic compound is due to which of the following.
Ionic compounds form crystals typically have. Other physical properties of ionic compounds. Ionic compounds have high melting and boiling points.
Ionic compounds form crystals. In a giant ionic lattice there are strong electrostatic forces of attraction acting in all. They are brittle.
Properties of Ionic Compounds Answers 1 Explain why ionic compounds have such high melting and boiling points when compared with covalent compounds. Properties of ionic compounds. Additionally when you stack these ions.
Most ionic compounds are solids that typically melt at high temperatures and boil at even higher temperatures. 1Every positive ion is surrounded by negative ions - GIANT IONIC LATTICE2This means they have very strong electrostatic forces of attraction whic. Properties Shared by Ionic Compounds They form crystals.
If the solid is heated until it melts the liquid will conduct electricity because the ions are mobile they can move. They form crystals They have higher enthalpies of fusion and vaporization than molecular compounds. Select all that apply.
Ionic compounds often have higher melting point than metals. Here is a short list of main properties. As a result of how ionic compounds form their general properties include the following.
Ionic compounds hold a strong force of attraction between the positive and negative. These compounds are formed through sharing of electrons and ionic compounds are formed by transferring of electrons. They have high melting points and also high boiling points.
ND MANI MORE Advertisement. As a result ionic compounds are usually soluble in water. The sharing of electrons electrostatic forces between ions of opposite charges o the attraction of anions to one another the number of neutrons in each atom.
The electrostatic forces of attraction between oppositely charged ions lead to the formation of ions. Properties of Ionic and Covalent Compounds One way of classifying chemical compounds is by whether they contain ionic bonds or covalent bonds. They have higher.
Solid ionic compounds do not conduct electricity because the ions are not free to move. These compounds are brittle and break into small pieces easily. The ions are easily hydrated by water molecules to form hydrated ions.
All ionic compounds are solid at room temperature. Ionic Compound A compound that contains two or more ions and is held together by ionic bonds. Ionic compounds are composed of ions.
High temperatures are required to overcome the attraction between. For the most part ionic compounds contain a metal bonded to a nonmetal. They are usually soluble in water and insoluble in solvents such as petrol kerosene etc.
THE MAIN PROPERTIES OF IONIC COMPOUNDS ARE AS FOLLOWS. High melting points and boiling points Ionic compounds are solids at. Monoatomic Ion - Consisting of one atom Polyatomic Ion -.
Thus electrons in the covalent compound are less mobile. The solubility of ionic compounds in organic solvents can be explained as below. They have high melting points and high boiling points.
Ionic compounds form crystal lattices rather than amorphous solids. Ionic compounds are basically defined as being compounds where two or more ions are held next to each other by electrical attraction. They are generally made up of two electrons which are shared by two atoms.
Properties of ionic compounds Ionic compounds have regular structures called giant ionic lattices. These compounds are brittle and break into small pieces easily. The four properties of Ionic compounds are.
Covalent compounds break apart into molecules when dissolving in water because they are non-polar. Ionic compounds form crystal lattices rather than amorphous solids. The physical properties of ionic compounds can be explained by thinking about their structure and bonding.
Ionic compounds form crystals.

Properties Of Ionic Compounds Chemistry Quiz Quizizz

Chapter 6 Ionic Compounds Ppt Video Online Download
8 9 Physical Properties Of Ionic Compounds Chemistry Libretexts

Comments
Post a Comment